Important: Please complete the Sexual Harassment Online Module ASAP!
Resuscitative Transesophageal echocardiography (TEE)
TEE allows the emergency physician to maintain the standard of an ultrasound-informed resuscitation in the scenario of cardiac arrest, where TTE is significantly limited.
Focused or resuscitative TEE (4 views) differ from comprehensive TEE (>20 views) that cardiology performs in that it is employed to identify specific questions.
TEE allows for potentially shorter chest compression pauses
TEE allows for evaluation for the quality of chest compressions
TEE allows for visualization of fine V-fib not seen on the monitor
Indications: Cardiac arrest (ACEP)
Contraindications: Esophageal injury or stricture and lack of a definitive airway
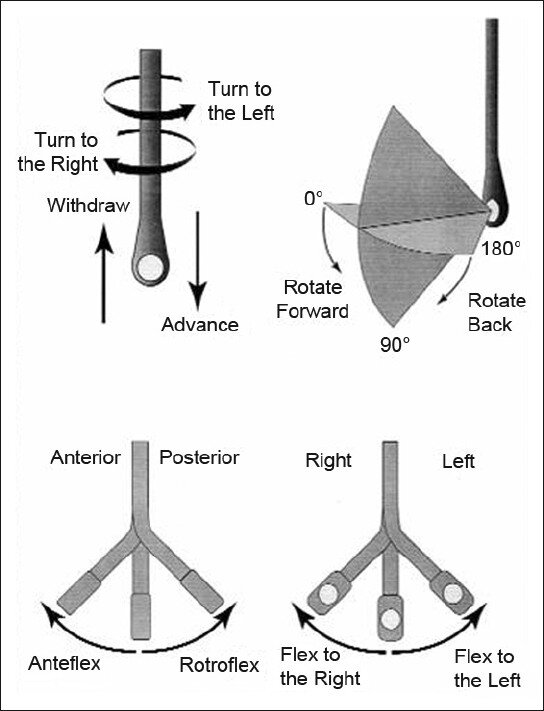
How to manipulate a TEE Probe:
5 different ways you can physically manipulate the TEE probe
1. Withdraw or Advance up or down patient’s esophagus
2. Turn probe to right or left
3. Turn tip of flip in anterior- ante-flexing or in the posterior direction called retro-flexing --> large wheel
4. Turn tip to Left or right --> small wheel (not typically used for our purposes)
5. In addition, you can rotate the transducer housed within the probe itself (AKA omniplane or multiplane)--> adjusts the beam angle anywhere between 0° and 180° --> two smaller buttons ( crystal rotation)
The views are obtained in the following order: :
- The midesophageal 4-chamber view (ME 4C) is obtained by advancing the TEE probe to the thoracic esophagus and orienting the multiplane at 0-20° in neutral flexion. You may need to retroflex slightly to see all four chambers.
- The midesophageal long-axis view (ME LAX) is obtained by leaving the probe in the same location as the midesophageal 4-chamber, but increasing the multiplane to between 110° and 160° while in neutral flexion.
- The transgastric short axis view (TG- SAX) is obtained by first moving the multiplane to 0°, then advancing the probe into the stomach and ante-flexing the probe
- The bicaval view (ME bicaval) is obtained by turning the entire probe to the patient’s right towards the superior vena cava (SVC) and inferior vena cava (IVC) while in the mid-esophagus, keeping the multiplane at 90-100° with neutral flexion
( The first 3 views are recommended by ACEP. Bicaval not recommended by ACEP)
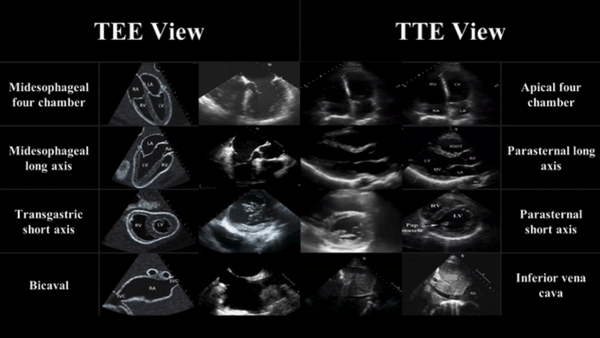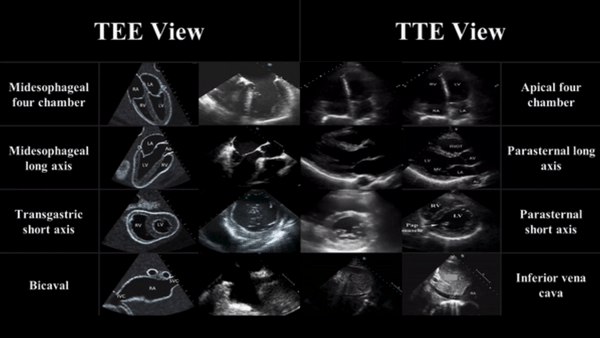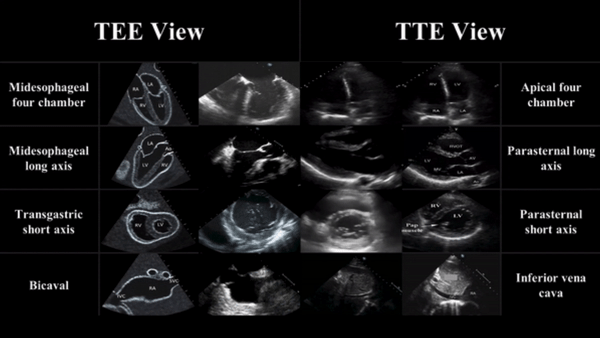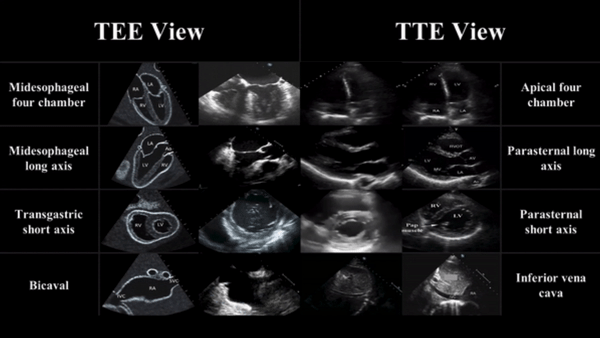
TEE views and their analogous TTE views
Midesophageal four chamber view (ME 4C)
- Apical four chamber view
- Great visualization of all chambers as well as the tricuspid and mitral valves in one plane.
- Evaluation of right and left ventricular systolic function and size
- Preferred view to evaluate for the presence or absence of a perfusing rhythm during a pulse check.
Midesophageal Aortic Long Axis view (ME LAX)
- Midesophageal analogous to the parasternal long axis view in TTE
- View includes the mitral and aortic valves, as well as the left atrium, left ventricle, and left ventricular outflow tract of the right ventricle.
- Evaluate left ventricular systolic function, and provides feedback on compression adequacy and location. High-quality compressions cause maximal compression of the left ventricle and visualization of the aortic valve opening and closing indicating forward flow of blood. Poor quality compressions are seen over the aortic root and there is no valvular indication of forward flow.
Transgastric Short Axis view (TG- SAX)
- Analogous to the parasternal short axis TTE view
- Evaluate left ventricular systolic function, including any regional wall motion abnormalities
- Can evaluate for acute MI and the presence of septal flattening in this view
Mid Esophageal Bicaval View (ME bicaval)
- Analogous to the inferior vena cava view of TTE
- Transducer plane cuts through the left atrium (LA), right atrium (RA), IVC and SVC.
This view allows the operator to evaluate for hypovolemia, atrial size, and interatrial septum bowing.
- Aids in the placement of central venous catheters, transvenous pacemakers, or extracorporeal life support (ECMO) vascular cannulas by observing the initial wire placement in the vasculature
- Can aid inevaluation of fluid status to guide fluid resuscitation (looking at respiratory variation in SVC)
Pitfalls
- Compressions do not need to be stopped for TEE insertion. Additionally, the TEE can be left in the esophagus during defibrillation. The probe should be inserted or withdrawn while the tip is in neutral position, and not while the tip is flexed to avoid esophageal injury.
- Images should be optimized to avoid foreshortening of the ventricles and to include the appropriate structures for each view.
- Pericardial effusions must be taken into clinical context, as small effusions can cause tamponade if accumulated rapidly, while large effusions can be well tolerated if they accumulate slowly.
- Clotted hemopericardium may be isoechoic with the myocardium, making it difficult to identify.
- Right ventricular failure is not specific to pulmonary embolism, and can be due to pulmonary hypertension or other etiologies such as right sided myocardial infarction, or even cardiac arrest itself.
- Pleural effusions can be mistaken for pericardial effusions. Multiple views should be used to corroborate findings.
- Fat pads can be mistaken for pericardial effusions, but these are hypoechoic rather than anechoic and limited to the anterior and apical regions of the heart, not circumferential.
Resource:
Check out this 3D module that you can practice on
https://pie.med.utoronto.ca/TEE/TEE_content/TEE_standardViews_intro.html
References:
Drs Lawrence Haines, Judy Lin and Alyssa Phuoc-Ngyuyen
Images: Adapted from Arntfield R, Pace J, McLeod S, et al. Focused transesophageal echocardiography for emergency physicians-description and results from simulation training of a structured four-view examination. Crit Ultrasound J. 2015;7(1):27.
Teran, Felipe, et al. "Evaluation of out-of-hospital cardiac arrest using transesophageal echocardiography in the emergency department." Resuscitation 137 (2019): 140-147.
ACEP policy statement