Avid learners:
As promised: part 2 on electrophysiology. The emphasis here will be modes of pacing and what they mean. Please note, I am not an electrophysiologist,
so if anything here is wrong, please respond ALL.
This topic may require multiple reads, and I encourage you to seek out other sources.
Please take this POD with a grain of salt.
Electrophysiology super-fellowships recently were extended to 2 years because this is a complicated topic.
We will focus on what the EP provider needs to know.
Typically a pacing mode is described in letters. For simplicity sake, we will stick to the old fashioned 3 letters.
A typical appearance is VVI [OO]. Most pacemakers will be reported with an omission of IV and V and will be just be the first 3 letters out of 5, in this case VVI.
Position 1 is what is Paced
Position 2 is what is Sensed
Position 3 is what is the action of the device.
Position 4 is rate modulation
Position 5 is for atrial, ventricular, or both pacing
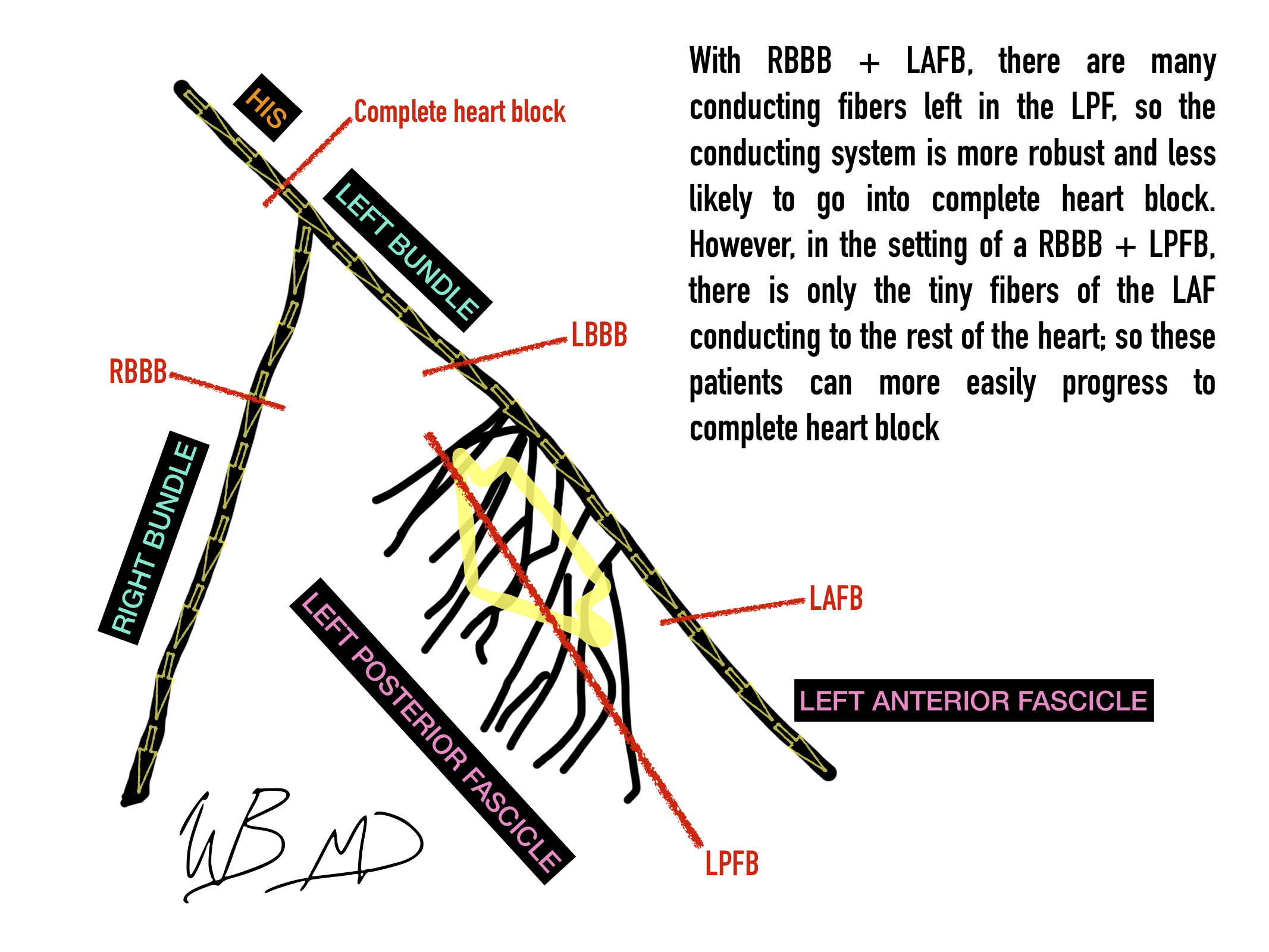
For this case (VVI), the ventricle is paced. The ventricle is also sensed, and if the pacemaker senses a ventricular beat, the pacemaker’s delivered beat will be inhibited. So: if the rate of the pacemaker is set to 50 bpm, and the ventricle is beating at 60 bpm without pacing, every sensed beat actually inhibits the pacemaker from firing. However, if the ventricular rate all of sudden drops to 25, there are half the beats to sense, and the pacemaker will then start pacing.
In short, for patients with an old school unipolar lead in the RV (VVI), if the ventricle does not beat faster than the programmed rate, the transvenous pacer will depolarize the RV. As I mentioned in my previous POD (Electrophysiology 101), this is why we see a LBBB morphology.
Here is a key:
reproduced from above source.
As per my review of the literature; the most confusing thing about pacing schema is the 3rd position:
If a pacemaker is in DDD mode (most common mode), it will pace both chambers (if necessary) AND senses both chambers. So, if the atria does not spontaneously fire at the programmed rate, it will trigger an electrode signal causing pacing the atria; and subsequently, it will do the same for the ventricle. If an atrial depolarization occurs spontaneously, it will ensure there is a subsequent atrial beat. In short, DDD mode automatically senses which chamber(s) is/are not firing and makes them contract in synchrony.
If a pacemaker is in DDI mode, “AV synchrony is provided only when the atrial chamber is paced.” Basically, this means the if the atrial chamber is not being paced because it is depolarizing on its own, the pacemaker will not depolarize the ventricle(s). But, if the atrial chamber needs to be paced, it will also subsequently pace the ventricle.
As mentioned previously in my POD, Cardiac Synchronization Therapy (CRT) expands the pacing to both ventricles in an attempt to improve cardiac output in the setting of reduced ejection fraction.
By far the most important mode of pacing that we utilize emergently in the ED is VOO. In this mode we just overdrive pace the ventricle. Typically these are patients with bradycardia and/or hypotension/AMS. Occasionally, you may see intermittent asystolic events due to profound heart block. Both of these patients need to have transvenous pacing until electrophysiology can place a permanent pacemaker. You can let them figure out the mode that best suits them.
Penultimate final point for patients with a pacemaker who come in unstable: if you a put a magnet on the pacemaker it will default the pacemaker to AOO, VOO, or DOO (all overdrive pacing). No response to this means dead battery or lead displacement, which means they need a revision.
Final point: as I mentioned in my last POD on electrophysiology, putting a magnet on a pacemaker is substantially different than putting a magnet on an AICD.
Magnet on pacemaker=overdrive mode
Magnet on AICD=inhibition of defibrillator